
Advancing
Global Health
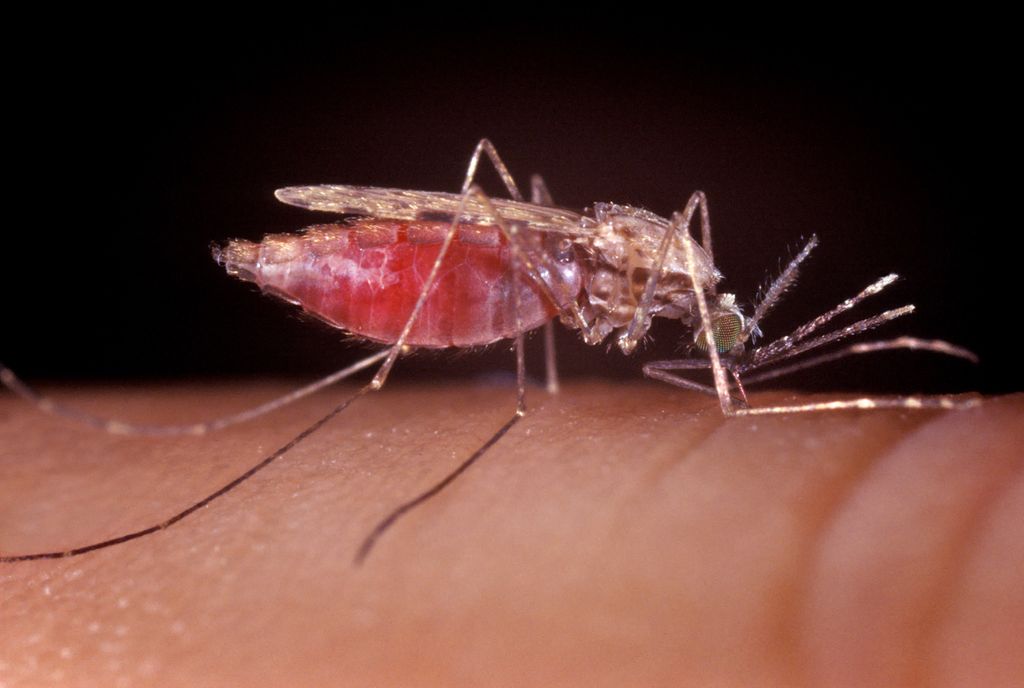
In the midst of an already-stalled global struggle against malaria, COVID-19-related disruptions to health care systems prompted a dangerous resurgence of this deadly disease in 2020. Recognizing the urgent need for new strategies, in May 2021 the World Health Organization (WHO) — with support from the FNIH’s GeneConvene Global Collaborativeopens in a new window — published an updated guidance frameworkopens in a new window to inform research and development of genetically modified mosquitoes.
Modifying the genes of malaria-carrying mosquitoes can reduce their ability to reproduce, so that there are fewer to spread the disease, or decrease the mosquitoes’ ability to transmit the malaria parasite. If proven safe, effective, and affordable, these technologies could offer a game-changing addition to the existing arsenal of interventions against malaria. But there are many important questions for decision makers to address when evaluating whether and how to move forward with research and implementation.
The FNIH-led GeneConvene initiative works to ensure that all stakeholders — including researchers, policymakers, regulators, and communities affected by malaria — are equipped with the right tools to make informed, responsible decisions about these technologies. The new WHO guidance is a significant milestone in this effort. It provides answers to some of the toughest questions in the field, including:
- How can the potential effectiveness and risks of genetically modified mosquitoes on reducing disease be evaluated?
- What are the key ethical considerations?
- Which regulatory frameworks will oversee decisions about research?
Like many other FNIH collaborations, GeneConvene centers on a critical inflection point where advances in biomedical research depend on bringing diverse groups together to pursue a common goal. With malaria causing more than 600,000 deaths worldwide in 2020 alone, there is an urgent need to evaluate the promise of genetically modified mosquitoes both quickly and responsibly.