Accelerating New Treatments ,
Delivering Impact
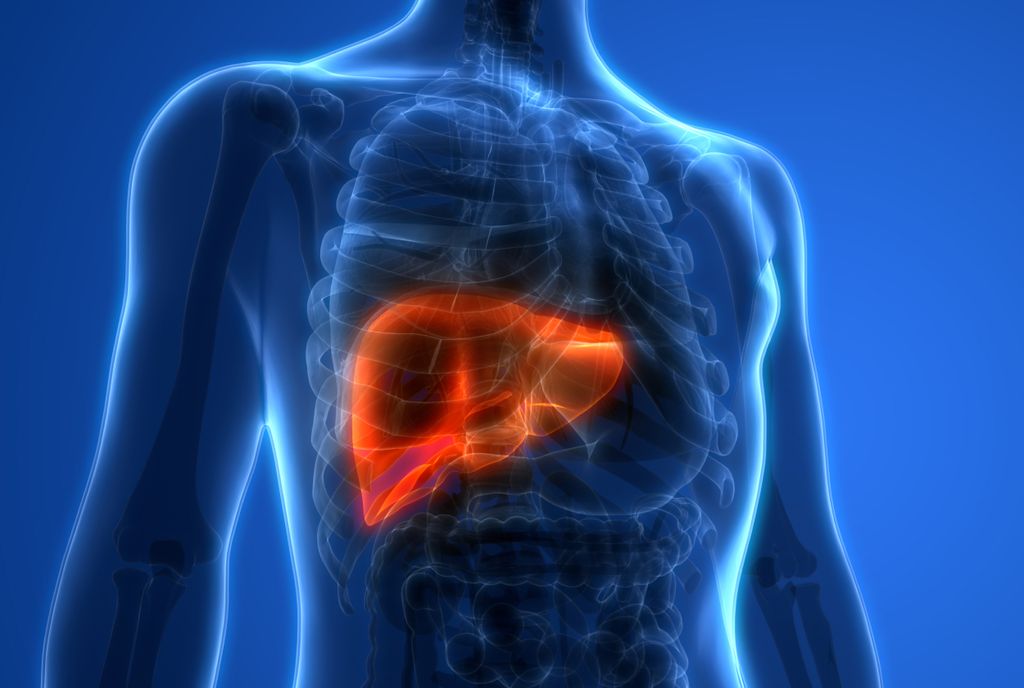
THE PROMISE:
Non-alcoholic steatohepatitis (NASH) is a serious liver disease estimated to affect between 9 and 15 million people in the U.S. The disease often remains undiagnosed in its early stages and can require a liver transplant or cause liver cancer — and ultimately death. Currently, diagnosing early-stage NASH requires a liver biopsy: a painful, invasive, and expensive process. In November 2021, the FNIH Biomarkers Consortium released the initial results from its NIMBLE project, which seeks to assess the suitability of several non-invasive, blood-based biomarkers for use in clinical trials of treatments for NASH. Several of these biomarkers did a better job of identifying patients at risk of developing NASH or progressing to cirrhosis than current standard tests and compared favorably with the diagnostic performance of liver biopsy, the current reference standard.
THE IMPACT:
Although further confirmatory study may be required, the new biomarkers examined by NIMBLE could potentially replace the need for patients who have or who may be at risk for NASH to undergo biopsies. If approved by the FDA, tests using these new markers could enable early and accurate diagnosis of at-risk patients, improving the quality of care, accelerating drug development, and reducing the burden imposed by current clinical care practices.
THE PROMISE:
Immunotherapies — treatments that stimulate the body’s own immune system to fight cancer — have proven to be an effective and promising therapeutic option for certain cancers in recent years. Yet these immune treatments do not work for all patients, and we lack a precise understanding of why. Developing standardized biomarker tests to understand how immunotherapies work in some patients, and thus to predict patient responses to treatment, is urgently needed for these therapies to benefit the maximum number of people.
PACT, a five-year public-private research collaboration launched in 2019 by NIH, the FNIH, FDA, and 12 pharmaceutical companies as part of the Cancer Moonshot, integrates the expertise of researchers at four top cancer research centers — Dana-Farber Cancer Institute, Stanford University, MD Anderson Cancer Center, and Mount Sinai Medical Center — to develop these assays and harmonize them for use across the cancer field.
THE IMPACT:
To date, PACT researchers have analyzed over 5000 samples from 12 clinical trials in different cancers and used the resulting data to validate existing assays across all four laboratories. Six existing assays commonly used in assessing immunotherapies and combination therapies across 15 different cancers have been harmonized to date. The resulting standards have been published in four major journal articles to make sure these tests can be performed uniformly in laboratories across the U.S. As data anticipated from an additional 35 trials becomes available, PACT researchers will be able to use it to help develop new biomarkers to help physicians select the most optimal immunotherapy treatments for their patients.

THE PROMISE:
In April 2021, the FNIH Biomarkers Consortium launched its Mucosal Healing in Ulcerative Colitis (UC) Project to improve diagnosis and treatment of a debilitating inflammatory bowel disease that affects more than three million people worldwide. This three-year initiative aims to generate best practices and consensus standards for assessing disease activity at the tissue-level in UC clinical trials. The program also hopes to develop cutting-edge methodologies to measure mucosal healing — the body’s ability to restore intestinal lining damaged by UC, and an important outcome in clinical trials — more accurately in the future.
THE IMPACT:
Creating a more precise measure with which to judge the outcome of clinical trials promises to meaningfully accelerate the development of new therapies for patients with UC. The ability to use a common protocol and automate the evaluation of mucosal inflammation and healing will enable these measurements to be applied more consistently, precisely, and rapidly and may reduce the number of biopsies needed. The goal is to advance physicians’ ability to help guide appropriate treatment to reduce or even prevent relapses and complications, improving the patient experience.